Toronto, Ontario Jul 3, 2025 (Issuewire.com) - A smarter, faster, more flexible way to manage compliance—new features empower MedTech teams to automate risk, capture QMS events, and streamline audits.
qmsWrapper, a leading provider of Quality Management Software for medical device companies, today announced an update that redefines how MedTech organizations manage compliance under MDR, IVDR, and the upcoming QMSR regulations.
This release introduces a risk-based event capture system, advanced form automation, custom process logs, and a centralized Hazard Log—all designed to make compliance smarter, faster, and truly scalable. From startups to growing manufacturers, teams now have access to enterprise-grade automation with unmatched flexibility.
“I talk with MedTech organizations every day—each has a different story about where they’re coming from and why they’re switching to qmsWrapper,” said Timea Torok, Account Liaison at qmsWrapper. “Too many QMS tools make compliance harder, not easier. They lock users into rigid workflows and hold their data hostage. Our goal is to give MedTech teams a truly configurable platform—with all the power of predefined modules and workflows, but the freedom to customize every aspect to fit their specific needs.”
With this update, we’ve added Events, Custom Logs, and Automated Follow-up Actions tied to forms. Our ecosystem keeps expanding in flexibility—while boosting speed, traceability, and accuracy—the three things every development team needs to succeed.
Key Features at a Glance
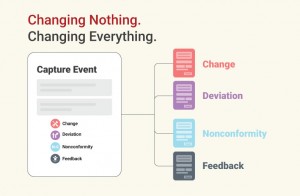
- Predefined Quality Events
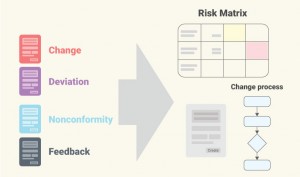
Instantly capture Change, Deviation, Non-Conformity, and Feedback with built-in flows that include integrated risk evaluation and follow-up automation. - Hazard Log for ISO 14971 Compliance
Centralize risk management in one place—traceable, audit-ready, and aligned with global regulatory standards. - Custom Logs & Event Modules
Create your event tracking systems using custom forms—ideal for unique company processes. Unlike other QMS software, qmsWrapper lets you build your modules inside the platform. - Smart Form Automation
Turn any form into a dynamic workflow. Trigger tasks, processes, risk assessments, or follow-up forms automatically—based on real-time field inputs like checkbox selections or status changes. - Connected, Prefilled Workflows
Forms and events flow in a defined sequence. Data from one step carries over automatically into the next, minimizing manual input and eliminating errors. - Action-Ready Events
Every event becomes a trigger. Automate task creation, initiate processes, or launch new forms—ensuring no risk or issue is left unresolved.
A Fully Integrated, Fully Automated Ecosystem
Unlike other QMS platforms, qmsWrapper is not just a collection of tools—it’s a unified ecosystem. Modules for CAPA, Change Management, Deviation Management, Non-Conformity, Training, Document Control, Audit Management, Project Management, QMS Management, Traceability Matrix, Customer Feedback, Supplier Management, Risk Management, Hazard Logs, Custom Logs, Automated SOP flows, Predefined Templates, and even a built-in Conversation Module are deeply integrated and fully automated.
Best of all? Everything is customizable—from workflows and forms to entire module structures. No other QMS platform gives MedTech teams this level of control and power in one cohesive system.
The new version is available now, fully compatible with existing qmsWrapper installations. No disruption—just smarter quality. Learn more or schedule a demo: https://store.qmswrapper.com/
Media Contact:
Nena Scot
Marketing & Communications
qmsWrapper
contact@qmswrapper.com
Media Contact
qmsWrapper contact@qmswrapper.com https://www.qmswrapper.com/