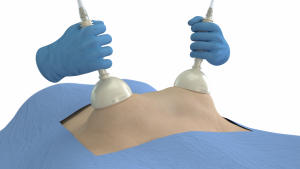
Fayetteville, Arkansas Oct 25, 2022 (Issuewire.com) - Lapovations, a medical device company creating a platform of innovative products to improve laparoscopy, is announcing the national launch of AbGrab® following the completion of a 225-patient, three-phase, multi-site human trial that demonstrated 98.7% reliability. AbGrab® is a revolutionary device that allows surgeons to lift the abdominal wall more reliably and less invasively at the beginning of laparoscopy or minimally invasive surgery of the abdomen.
AbGrab® was developed primarily using non-dilutive grant funding from the National Science Foundation and Arkansas Economic Development Commission, non-dilutive prize money from national business plan competitions, and investments from several startups and early-stage development incubators.
AbGrab® was registered with the FDA in 2021, after which the company conducted a pre-launch trialing program that resulted in 10 surgeons using the product in over 150 laparoscopies. “Overall reception has been fantastic, with 100% of trial surgeons indicating they plan to continue using AbGrab once we launch nationally” said CEO Jared Greer. “The most important user needs we consistently hear from surgeons is reliability, and we’re thrilled AbGrab was able to demonstrate 98.7% reliability in our recently concluded trial.”
Chief Operating Officer Nhiem Cao led the engineering, testing, and manufacturing efforts for AbGrab®. “The evaluations we performed were incredibly rigorous and we took a methodical scientific approach to identify the best performing design”, said Cao. He continued, “most startups burn through a lot of capital developing their initial products, but our grant and other non-dilutive funding allowed us to drastically reduce the investor resources required to build, test, and finalize design surgeons love.”
This national launch comes on the heels of Lapovations signing agreements with seven distributors to sell AbGrab® across the United States. Collectively, these distributors have 22 representatives selling AbGrab® in territories covering 11 densely populated states, including Florida, Ohio, and Pennsylvania.
“There are several different sales and distribution strategies that can be employed for products like AbGrab, so choosing the right model was critical. The network of independent reps we’ve assembled maximizes our shots on goal, provides agility, and keeps overhead low”, said Chief Technology Officer and VP of Sales Spencer Jones. He continued, “We plan to continue aggressively expanding our rep network and aim to have at least 75 independent reps selling AbGrab across the U.S. in the next 12 months”.
Though often the shortest part of the procedure, laparoscopic abdominal entry accounts for ~50% of serious laparoscopic complications and litigations related to laparoscopy (1, 2). To reduce this risk, surgeons will lift the abdominal wall before entry, but current lifting techniques can be unreliable or invasive. AbGrab® uses suction to provide a more reliable, less invasive way to elevate the abdominal wall.
About Lapovations
Lapovations, LLC is a medical device company creating a platform of innovative products to improve laparoscopy, or minimally invasive surgery of the abdomen. The company’s first product, AbGrab®, is a novel device that uses suction to lift the abdominal wall at the start of the procedure. AbGrab® is more reliable and less invasive than current lifting methods. For more information contact: mediarelations@lapovations.com or visit www.lapovations.com
1. Thepsuwan, J., Huang, K., Wilamarta, M., Adlan, A., Manvelyan, V. and Lee, C., 2013. Principles of safe abdominal entry in laparoscopic gynecologic surgery. Gynecology and Minimally Invasive Therapy, 2(4), pp.105-109 [Accessed 1 September 2021].
2. Jansen, F., Kolkman, W., Bakkum, E., de Kroon, C., Trimbos-Kemper, T. and Trimbos, J., 2004. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. American Journal of Obstetrics and Gynecology, 190(3), pp.634-638 [Accessed 1 September 2021].
Media Contact
Lapovations mediarelations@lapovations.com 479-304-0436 700 W. Research Center Blvd., Suite 1437 http://www.lapovations.com